Shanghai, China --- the Toumai® Laparoscopic Surgical Robot (also known as endoscopic surgery robot) independently researched and developed by MicroPort® (Shanghai) Medbot Co., Ltd. (), a robotics-specialized subsidiary of Shanghai MicroPort® Medical (Group) Co., Ltd. (hereinafter referred to as "Microport®") passed the Special Procedure of National Medical Products Administration (NMPA) for Review of Innovative Medical Devices and entered the "Green Path”of approval procedure on October 17, 2019. Up to now, a total of 17 products of MicroPort® or related companies have been approved to enter the Special Procedure for Review of Innovative Medical Devices.
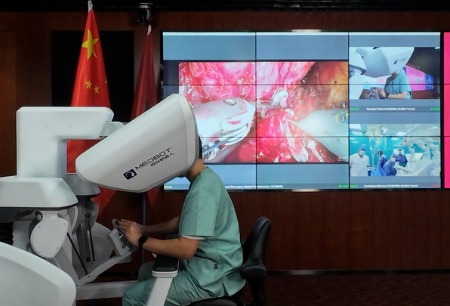
Toumai® Laparoscopic Surgical Robot approved this time is a endoscopic surgery robot product independently developed by Microport® Medbot, which consists of three parts: patient operation platform, image trolley and doctor console, which serve to assist in completing micro-port endoscopic cases, especially the difficult and complicated operations completed by open surgery or conventional laparoscopic surgery. The wrist surgical instruments of Toumai® Laparoscopic Surgical Robot are highly flexible, which can provide high dexterous exercise ability in narrow space and reduce the amount of intraoperative bleeding and the incidence of complications; the 3D celiac lens provides a 3D and real surgical view field, which assists in the complex and fine surgical operation under endoscopy; the intuitive master-slave teleoperation is sensitive and easy to use, thus simplifying the operation and shortening the surgical time. The product has high clinical value.
It is an independent innovation achieved of Toumai® Laparoscopic Surgical Robot in basic components and technologies such as robot body, 3D electronic laparoscopic system and robot control algorithm, and solved the "bottle neck" problems in the process of productization of a series of domestic surgical robots. Compared with similar imported products, Toumai® Endoscopic Surgery Robotic System optimizes the operator's surgical experience, reduces the cost of equipment maintenance and consumables, and reveals the "Chinese Intelligent Manufacturing" at the international leading level.
Early in 2014, MicroPort® began to lay out the field of medical robots and started the independent research and development of endoscopic surgery robots. From then on, its subsidiary company Microport® Medbot has focused on the research and development and industrialization of micro-port surgery robots, and gradually formed and improved multi-department integrated solutions represented by endoscopic surgery robots, joint replacement surgery robots and 3D electronic laparoscopy.
It is reported that there is no domestic endoscopic surgery robot product on the market at present. Toumai® Endoscopic Robot has been approved to enter the Special Procedure for Review of Innovative Medical Devices, which will speed up its marketization process in China, and the clinical trial to evaluate the clinical safety and effectiveness of Toumai® Endoscopic Robot will soon be launched in China, which will play a positive role in popularizing international cutting-edge technology and promoting the development of domestic related industries and is expected to benefit more patients.








 Hu ICP Bei No. 20013662 HGWA Bei No. 31011502015178
Hu ICP Bei No. 20013662 HGWA Bei No. 31011502015178 " are registered trademarks of Shanghai MicroPort Medical (Group) Co., Ltd.” . They have been authorized to be used by Shanghai Microport Medbot (Group) Co., Ltd., and no other party shall use such trademarks without prior written permission thereof.
" are registered trademarks of Shanghai MicroPort Medical (Group) Co., Ltd.” . They have been authorized to be used by Shanghai Microport Medbot (Group) Co., Ltd., and no other party shall use such trademarks without prior written permission thereof.
